November 21, 2017
Researchers at IST Austria define function of an enigmatic synaptic protein
Synaptotagmin 7 ensures efficiency of inhibitory signal transmission – Study published in Cell Reports
Communication is often mired in contradiction – also in the brain. Neuroscientists at IST Austria were now able to resolve one such contradiction.
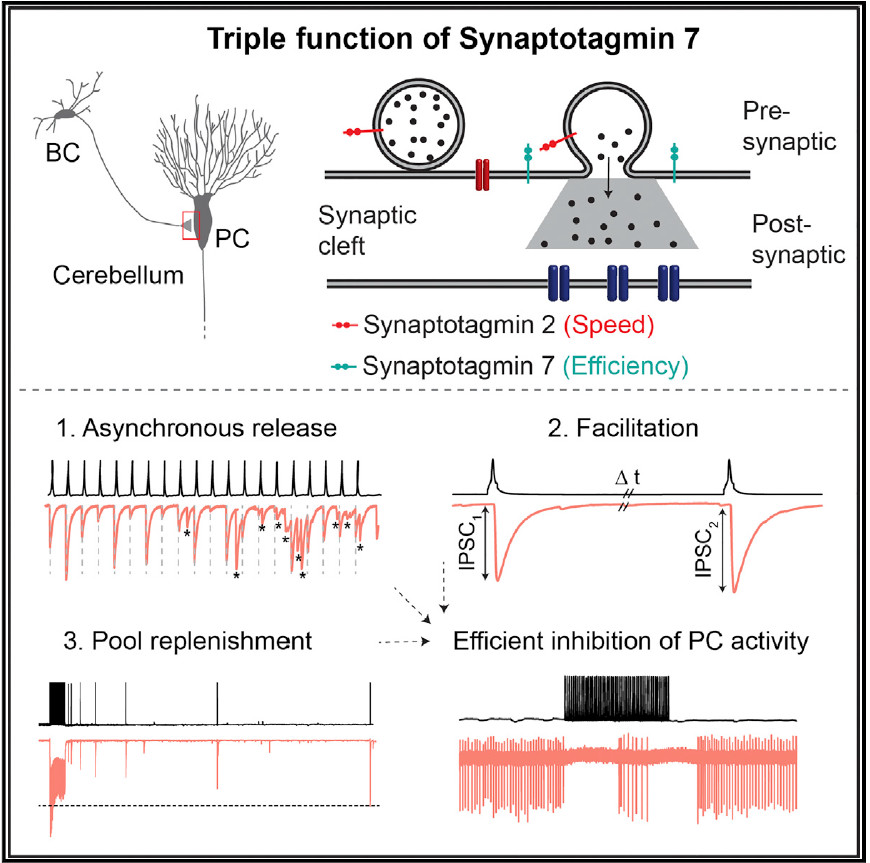
In our brains, neurons communicate by sending chemical signals across their connections, the synapses. The molecular machinery required to send a signal involves not only the signal itself, the neurotransmitter, but a large variety of other proteins that act as sensors, effectors, modulators, and scaffolds. Synaptotagmins are part of this complex machinery. Synaptotagmin proteins come in many flavours: humans and other mammals have 17 different varieties. For most of these proteins, however, scientists do not yet understand the function. A group of neuroscientists led by Peter Jonas, Professor at the Institute of Science and Technology Austria (IST Austria), have now resolved the role of Synaptotagmin 7 during signal transmission at an inhibitory synapse. Writing in Cell Reports, the team, including first author and PhD student Chong Chen as well as researchers at the Max Planck Florida Institute for Neuroscience, shows that Synaptotagmin 7 ensures the efficiency of high-frequency inhibitory synaptic transmission. “The role of Synaptotagmin 7 has been controversial. For the first time, we defined its functional contribution at an inhibitory GABAergic synapse”, explains lead author Peter Jonas.
Conflicting role in signal transmission
Only in January of this year, Jonas and Chen showed that Synaptotagmin 2 is the calcium sensor that makes certain synapses fast – those that use the transmitter GABA. In the current study, the researchers turned their attention to another member of the Synaptotagmin family, Synaptotagmin 7. The brain contains a large amount of Synaptotagmin 7, but so far, scientists were not able to pinpoint the protein’s function. Partly, the reason is a contradiction between the function Synaptotagmin 7 seemed to have, and the characteristics of signal transmission observed.
Synaptotagmin 7 appears to function as a calcium sensor that mediates the release of a variable barrage of neurotransmitter into the synaptic cleft, a phenomenon called asynchronous transmitter release. There are also indications that Synaptotagmin 7 plays a role in facilitation, an increase in neurotransmission at the synapse. But on the other hand Synaptotagmin 7 is also found in large amounts in a class of neurons called fast-spiking, parvalbumin-expressing GABAergic interneurons. These neurons form synapses which appear to contradict the suggested functions of Synaptotagmin 7: the synapses release neurotransmitter in a tightly synchronized manner, rather than asynchronously, and show a reduction in neurotransmission, rather than facilitation during repetitive stimulation. In their study, Chen et al. now resolve this apparent contradiction.
Synaptotagmin 7 regulates information flow in cerebellum
The researchers investigated how signal transmission changes when they delete Synaptotagmin 7 in an inhibitory synapse, the GABAergic synapse between the basket cells (BCs) and Purkinje cells (PCs) in the cerebellum (a brain region required for motor control). They show that, indeed, the function of Synaptotagmin 7 is to contribute to asynchronous transmitter release, replenishment of vesicles containing neurotransmitter, and facilitation. But rather than being mutually exclusive, these three functions appear to coexist at BC-PC synapses. Elucidating the effects of Synaptotagmin 7 required careful analysis, because asynchronous release is small and facilitation is overlaid by depression. However the authors demonstrate a substantial difference in the extent of depression. Little depression occurred in the presence of Synaptotagmin 7, but a lot of depression was found in the absence of Synaptotagmin 7. Thus, Synaptotagmin 7 ensures the efficient and frequency-independent signal transmission at the BC-PC synapse, one of its fundamental properties.
When the researchers looked at the level of neuronal networks, they found that Synaptotagmin 7 allows single basket cells to control the activity of a Purkinje cell. These neurons are the only ones that send information out of the cerebellum. Synaptotagmin 7 therefore has a strategic position to regulate the flow of information in this motor circuit. The researchers also found that Synaptotagmin 7 plays a similar (albeit quantitatively smaller) role at BC synapses in the hippocampus, a seahorse-shaped brain region involved in memory and spatial coding. Peter Jonas summarizes: “We have identified a critical role for Synaptotagmin 7 in maintaining the efficacy of transmission at GABAergic synapses in the cerebellum and hippocampus.”
Author biographies
Peter Jonas seeks to understand the function of neuronal microcircuits. His group quantitatively addresses the mechanisms of synaptic signalling at inhibitory synapses releasing GABA. Among other awards, Peter Jonas received the Wittgenstein Award 2016, two ERC Advanced Grants, and the Gottfried Wilhelm Leibniz Award. He is Member of the Academia Europaea and the German Academy of Sciences Leopoldina. Peter Jonas joined IST Austria in 2010. Chong Chen is a PhD student in the group of Peter Jonas. He joined IST Austria’s interdisciplinary graduate school in 2014. Before he came to IST, he earned a master of medicine degree at the School of Medicine, Shanghai Jiaotong University.
Publication
Chen, Chong et al. 2017. Triple function of synaptotagmin 7 ensures efficiency of high-frequency transmission at central GABAergic synapses. Cell Reports. DOI: 10.1016/j.celrep.2017.10.122