January 9, 2020
„Flash and freeze“ reveals dynamics of nerve connections
New method to simultaneously study both structure and function of synapses in intact brain circuitry of mice presented – Study published in Neuron
© Carolina Borges Merjane
How do the physical parts of a neuron enable it to process and communicate information? Relating structure to function is a long-standing goal in neuroscience. In the latest issue of the journal Neuron, the group of Peter Jonas, professor at the Institute of Science and Technology Austria (IST Austria), reports a novel method to achieve this objective.
Uniting structure and function of synapses is challenging: Function is studied in living tissue, measuring electrical signals at millisecond precision with electrophysiology, while the observation of fine structure at nanometer scale requires tissue to be fixed for electron microscopy. Peter Jonas, professor at the Institute of Science and Technology Austria (IST Austria), and his group members, first authors Carolina Borges-Merjane (postdoc) and Olena Kim (PhD student), have developed a so-called “flash and freeze” method for studying structure and function of synapses in intact neural circuits in mammalian brain slices.
Method makes structural changes during signaling visible
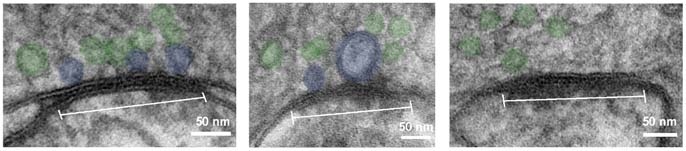
“Flash and freeze” refers to the flash of light used to stimulate the neurons, followed by immediate freezing of the tissue to fix it in its most native state. Peter Jonas sums up the challenge: „We mostly do impossible experiments in the Jonas lab, and the new research falls exactly into this category. Here, we take a synapse, stimulate it with light and, within milliseconds, shoot it into a chamber that freezes the structure at minus 196 degrees Celsius and at a pressure of 2,000 bar”. The sample is then dropped into a tank of liquid nitrogen and prepared for analysis by electron microscopy.
This set-up allows neuroscientists to stimulate neurons and freeze the tissue immediately afterwards for analysis by electron microscopy, so that changes in anatomy right after stimulation become visible. “It is a very dynamic way of studying synapses”, explains Carolina Borges-Merjane, “we can flash and then freeze immediately or wait a few milliseconds or even seconds. By taking several such snapshots, we reveal the time course of structural changes that happen during synaptic transmission.” In a parallel series of electrophysiology experiments in living tissue, the researchers characterized the functional dynamics of the same type of synapses. By integrating these data sets, they show how structural changes give rise to the observed function.
Function retained in intact networks
The method presented by the Jonas group is a modification of the “flash and freeze” protocol initially used for studying neurons of the worm Caenorhabditis elegans and individually isolated or dissociated mammalian neurons. The difference: the newly reported method uses slices of the mouse brain, in which neuronal networks remain largely intact and alive. “The function of neurons is usually studied in slices of brain tissue, in which networks remain intact. Synaptic structure is typically studied in chemically fixed samples or, as previously done with flash and freeze, with dissociated neurons. With our method, we can now use the same type of preparation used to study synaptic function to simultaneously study structure”, Olena Kim points out. The authors also demonstrated that the method is widely applicable to different brain regions and can therefore be used in studies of a variety of synapses in the brain.
Near identity of structurally and functionally defined vesicle pools demonstrated
In a proof-of-principle experiment, the researchers analyzed pools of vesicles at a cortical synapse. These vesicles contain the neurotransmitters that transfer signals to the neighboring neuron. They found that the structurally defined “docked” pool and the functionally defined “readily releasable pool” of synaptic vesicles are in fact revealed to be very nearly the same, once observed and analyzed using their new integrated method. “This has never been demonstrated directly. We interpret our results as meaning that vesicles fuse and integrate with the plasma membrane”, Jonas explains. “Our finding underlines how important it is to extend studies of both structure and function to cortical circuits.”
Publication
Carolina Borges-Merjane, Olena Kim & Peter Jonas. 2020. Functional electron microscopy (“flash and freeze”) of identified cortical synapses in acute brain slices. Neuron. DOI: 10.1016/j.neuron.2019.12.022
Funding information
This project has received funding from the European Research Council (ERC) and European Commission (EC) under the European Union’s Horizon 2020 research and innovation programme (ERC grant agreement no 692692 and Marie Sklodowska-Curie 708497) and from the Fonds zur Förderung der Wissenschaftlichen Forschung (Z 312-B27 Wittgenstein award and DK W 1205-B09).
Animal welfare
Understanding how the brain stores and processes information is only possible by studying the brains of animals while they carry out specific behaviours. No other methods, such as in vitro or in silico models, can serve as alternatives. The animals were raised, kept and treated according to the strict regulations of Austrian law.



