October 10, 2024
From Chaos to Structure
How a bunch of seemingly disorganized cells go on to form a robust embryo
Embryo development starts when a single egg cell is fertilized and starts dividing continuously. Initially a chaotic cluster, it gradually evolves into a highly organized structure. An international team of researchers including scientists from the Institute of Science and Technology Austria (ISTA) has provided new insights into the process, emphasizing the critical role of both chaos and order. The results are published in Science.

Pipetting liquids into tiny test tubes, analyzing huge datasets, poring over research publications—all these tasks are part of being a scientist. But breaking this routine is essential. Time away from the usual work environment can spark creative ideas. Lab retreats, for instance, offer a great setting where researchers can engage with other peers, often leading to new collaborations.
The latter was true for Bernat Corominas-Murtra and Edouard Hannezo from the Institute of Science and Technology Austria (ISTA). Fascinated by a dataset showcased during a poster session at a collaborative retreat research group in Spain, Corominas-Murtra started a lively discussion with fellow researcher Dimitri Fabrèges, a postdoc from the research group of Professor Takashi Hiiragi at the Hubrecht Institute in Utrecht, The Netherlands. What started as a conversation has now turned into a publication in Science.
The international team of researchers has built a comprehensive atlas of early mammalian morphogenesis—the process of an organism developing shape and structure—analyzing how mouse, rabbit, and monkey embryos develop in space and time. Based on this atlas, they see that individual events such as cell divisions and movements are highly chaotic, yet the embryos as a whole end up looking very similar to one another. With this dataset, they propose a physical model that explains how a mammalian embryo builds structure from chaos.
From one to many
In animals, embryonic development starts when an egg cell is fertilized. This event triggers an array of consecutive cell divisions, known as cleavages. In a nutshell, a single cell divides into two, then two become four, four become eight, and so forth. Eventually, the bulk of cells form into a very organized structure called the blastocyst, from which all future organs and tissues develop. The entire process is termed morphogenesis.
“These early steps of embryonic development are key, as they set the stage for all subsequent developmental processes,” explains Edouard Hannezo. In some animals, for instance, in C. elegans—a transparent roundworm and one of the most studied model organisms by developmental biologists—the divisions in the early embryo are extremely well regulated and orientated the same way across different embryos, giving rise to organisms that all have the same number of cells. In mammalian species, however, it seems like divisions are much more random, both in timing and orientation. This raises the question of how reproducible mammalian embryonic development proceeds despite this disorder.
A detailed embryo map
To address this question, the Hiiragi group set out to image and quantitatively analyze many different embryos, to compare their similarities both within and between different mammalian species, from mice to rabbits and monkeys. Dimitri Fabrèges and colleagues created a so-called ‘morphomap’—a map to visualize high-dimensional morphological data. “It’s an imaging analysis pipeline showing how embryos behave in time and space—a precise atlas of an embryo’s morphogenesis,” explains Hannezo.
The map allowed the scientists to quantitatively analyze the developmental process by addressing questions such as the inter-embryo variability of development. With this dataset, the scientists were able to define what ‘normal’ morphogenesis looks like.
Fabrèges presented the morphomap at the lab retreat in Spain. The data showed that the first divisions after fertilization were not regulated across mice, rabbits, and monkeys. The cells divided randomly until they reached the 8-cell stage, a stage where all embryos suddenly started to look the same. “After looking very different in the first stages, embryos seemed to converge toward each other’s shape at the end of the 8-cell stage,” Hannezo continues. But how come? What brings structure to this chaos?
An embryonal Rubik’s cube—cell cluster optimizes its packing
Corominas-Murtra and Hannezo, both theoretical physicists, were fascinated by this dataset and set out to understand this process from a theoretical standpoint.
However, an embryo’s shape is highly complex, making it difficult to determine what it means for two embryos to be similar or different. The scientists discovered that they could effectively approximate the full complexity of the structure of an embryo simply by studying the configurations of the cell-to-cell contacts. “We think that we can derive most of the important details about the morphology of an embryo by understanding the arrangements of cells or knowing which cells are physically connected—similar to connections in a social network. This approach significantly simplifies data analysis and comparisons between different embryos,” says Corominas-Murtra.
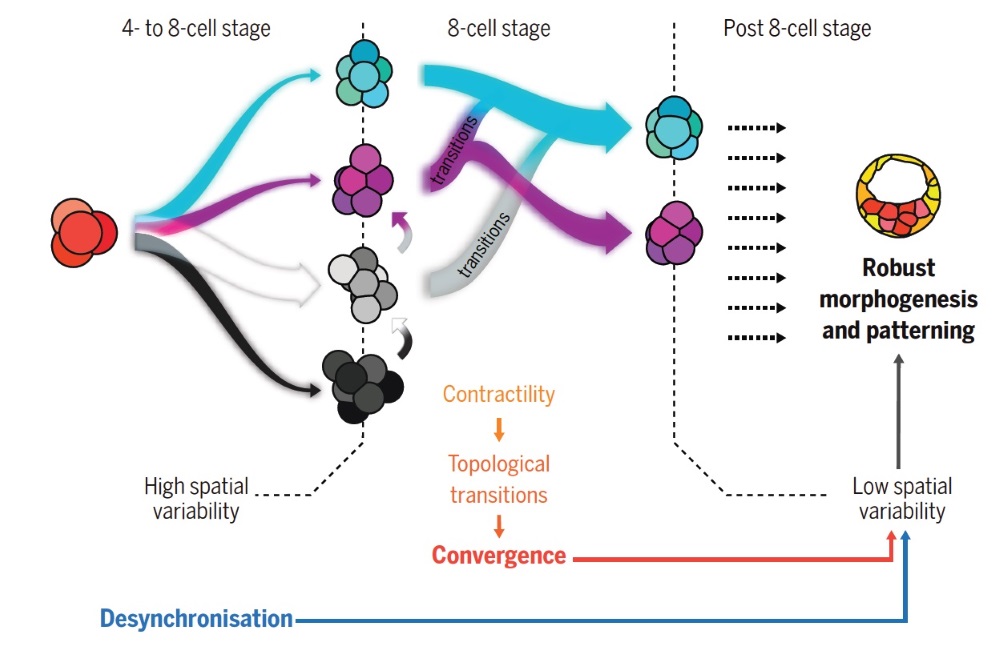
Using this information, the scientists created a simple physical model for how embryos converge to a reproducible shape. The model shows that physical laws drive embryos to form a specific morphology shared among mammals.
By destabilizing most cell arrangements except a few selective ones that lower the surface energy of the embryo, physical interactions between cells can guide the formation toward a defined shape. In other words, cells tend to stick more and more together and this seemingly simple process actually drives the embryo through successive rearrangements to the most optimal packing. “It’s like embryos solve their own Rubik’s cube,” says Corominas-Murtra.
No chaos, no structure
The results provide a detailed look at how the development of mammalian embryos is governed by variability and robustness. Without chaos, there is no structure; one needs the other. Both are essential parts of what constitutes ‘normal’ development. “We’re finally starting to have tools to analyze the variability of morphogenesis, which is crucial to understanding the mechanisms of developmental robustness,” Hannezo summarizes. “Randomness seems to be a primary force in the generation of complexity in the living world,” adds Corominas-Murtra.
By gaining more knowledge of what normal looks like, scientists also gain insights into abnormalities. This can be very helpful in areas, such as disease research, regenerative medicine, or fertility treatments. In the future, this knowledge can assist in selecting the healthiest embryo for in vitro fertilization (IVF), thereby improving the implantation success rate.

Publication:
D. Fabrèges, B. Corominas Murtra, P. Moghe, A. Kickuth, T. Ichikawa, C. Iwatani, T. Tsukiyama, N. Daniel, J. Gering, A. Stokkermans, A. Wolny, A. Kreshuk, V. Duranthon, V. Uhlmann, E. Hannezo, T. Hiiragi. 2024. Temporal variability and cell mechanics control robustness in mammalian embryogenesis. Science. DOI: 10.1126/science.adh1145
Funding information:
This project was supported by ISTA’s core funding to Edouard Hannezo.
Information on animal studies:
To better understand fundamental processes, for example, in the fields of neuroscience, immunology, or genetics, the use of animals in research is indispensable. No other methods, such as in silico models, can serve as an alternative. The animals are raised, kept, and treated according to the strict regulations of the respective countries. The research with animals was conducted in The Netherlands.



