March 12, 2020
Proteins as molecular switches
IST Austria biochemists identify self-organizing properties of Rab proteins – Study published in PNAS
© IST Austria – Loose Gruppe
A long-standing open question in the field of cell research is that of the self-organization properties of proteins called Rab small GTPases. It was now tackled by Urban Bezeljak, PhD student in the group of Martin Loose at the Institute of Science and Technology Austria (IST Austria), together with theoretical biophysicists from the National University of Singapore, in a current study published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS).
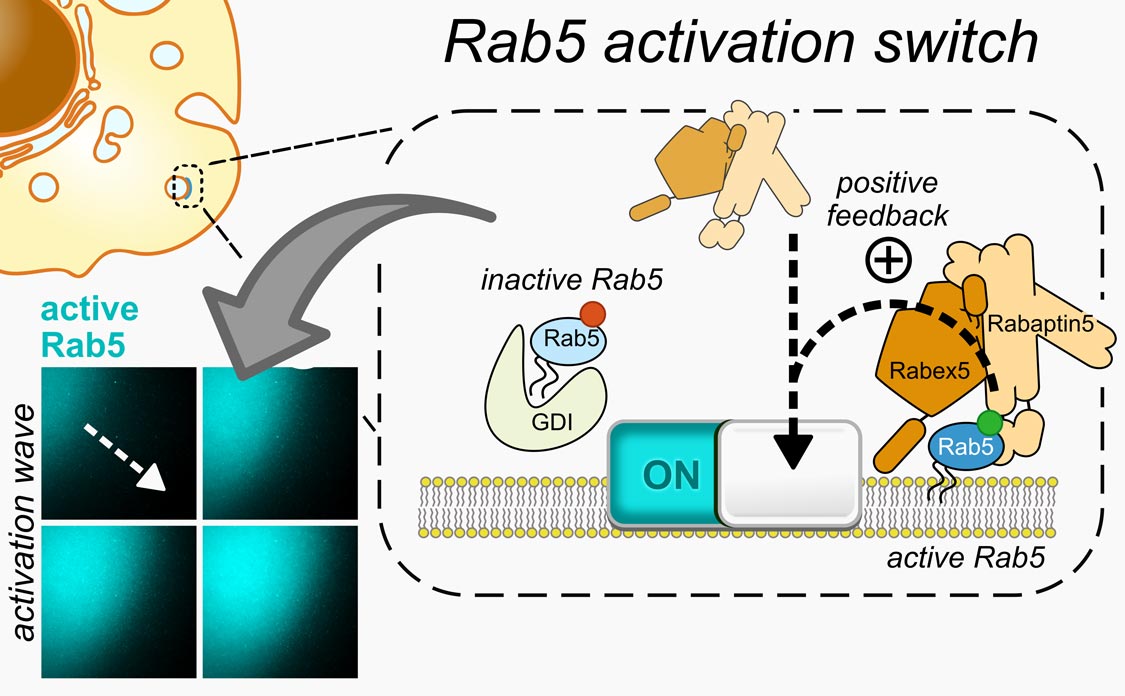
Rabs control precise funneling of different components inside our cells. The researchers dissected the details of this process by rebuilding it in a test tube, allowing them to directly observe the dynamics of fluorescently labeled proteins under a microscope. Bezeljak: “We found out that Rabs function like molecular switches, which can simultaneously switch on thanks to a positive feedback in their activation. This unique feature helps Rabs to orchestrate trafficking of membranes that encapsulate cellular cargo.“
Watch the corresponding video on YouTube
Biological membrane (dark), onto which the activated fluorescently labeled Rab5 (cyan) binds in a spreading wave-like pattern. This happens because active and membrane-bound Rab5 can recruit more fluorescent proteins of its kind, resulting in positive feedback and an activation wave as the proteins diffuse along the 2D membrane.
© IST Austria – Loose group
This study provides new insights into small GTPase networks that organize our cells in space and time and introduces a unique reconstitution approach, which can be used to study similar regulatory circuits. This understanding is especially important as these biochemical networks are commonly misregulated in cancer, neurodegenerative diseases and infection.
The collaborators from the lab of Timothy Saunders at the Mechanobiology Institute of the National University of Singapore Read have nicely summarized the research in this article.
About the author
Before joining IST Austria in 2015, Urban Bezeljak obtained his BSc and MSc in biochemistry at the University of Ljubljana, Slovenia. There, he entered the field of synthetic biology and worked on directed protein assembly and designed genetic networks. Urban was able to combine his interests in Martin Loose’s lab, where he is finishing his PhD on bottom-up examination of Rab GTPase regulation. In 2018, he received a Tuma Scholarship for exceptional Slovenian graduate students in Austria.

Urban Bezeljak, PhD student in the Loose group © IST Austria
Publication
Bezeljak U, Loya H, Kaczmarek B, Saunders TE & Loose M. 2020. Stochastic activation and bistability in a Rab GTPase regulatory network. PNAS. DOI: 10.1073/pnas.1921027117
Funding information
This work was supported by the Human Frontier Science Program (HFSP RGY0083/2016).



